"At the most basic level, competition in Health care must take place where value is actually created."
Michael E. Porter
Let us develop value for your project
Economic models
- Hospital prioritization
- Cost-effectiveness
- Burden of Disease
- Budget impact
Models
“Competition on value must revolve around results. The results that matter are patient outcomes per unit of cost.”
- Michael E. Porter
How health economic models benefit MedTech
How much does the disease or condition of interest cost the healthcare system? What is the impact of the disease on patients? Coreva Scientific helps you to answer these questions, giving you the ammunition to challenge payers and the evidence to undertake further analyses of your product’s cost effectiveness.
Healthcare should not be a race to the bottom on price! Value in healthcare is providing patients and providers with benefits at reasonable cost. How your product balances costs and effectiveness is important and something that all health technology assessment agencies want to know. Let Coreva Scientific design and build an analysis that is optimized for your product.
Not sure where to focus your sales team? Then a hospital prioritization tool should help. Using data on hospitals in your target area, the tool lets your sales team weight, sort, and rank hospital features to find their most appropriate target.
Purchase price is one thing, but how much will it actually cost a buyer to introduce your product? Is a reorganization of their workforce or additional training required? Will your product result in cost savings elsewhere? Coreva Scientific answers these questions as part of developing a budget-impact model.
See our work: Impact of product X on procedure Y: model example
Evidence communication
- Congress appearance
- Sales brochure
- Manuscripts
- White paper
- Slide deck
Evidence Communication
Congresses are great forgetting into direct communication with physicians, surgeons, nurses, general practitioners, hospital managers, and payers.
The average time reading a branded email is 10 seconds. For content aimed to generate interest, you have 5-30 seconds to grab your customers attention.
Want to tell a story? Need to communicate the success of other customers or describe benefits of your product that cannot be studied in a clinical trial? A white paper is likely what you need and the medical writers at Coreva Scientific can help you achieve it.
Internal or external communications often occur during a meeting or conference call. A slide deck supports you but does not distract from you. Coreva Scientific will help you balance the data with the messaging, staying on point, and remaining compliant.
When sales require a more in-depth but still user-friendly approach to communicate your product’s value, consider a sales brochure. All the key data from your dossier presented in easy reading sections.
Do you want to present the evidence for your product in a scientific setting while keeping it short and visually appealing? Appearances at congresses can get the word out there while a full peer-reviewed publication is being prepared. Your presence at a congress can come in different forms: abstract, symposium, poster and presentation.
Abstract
Having work accepted for presentation at a congress requires a short, informative, high-quality abstract. Coreva Scientific has the experience to produce these for you and create valuable exposure for your product.
Symposium
Symposia are industry organized, topic focused events that run alongside the main congress. Coreva Scientific are experienced at crafting and presenting the content for these events, ensuring that your product value messages come to the fore.
Poster and Presentation
Once an abstract is accepted the next step is to create a short, eye-catching way of communicating your key message. Whether it be a poster or oral presentation, Coreva Scientific can craft the messaging and presentation for you.
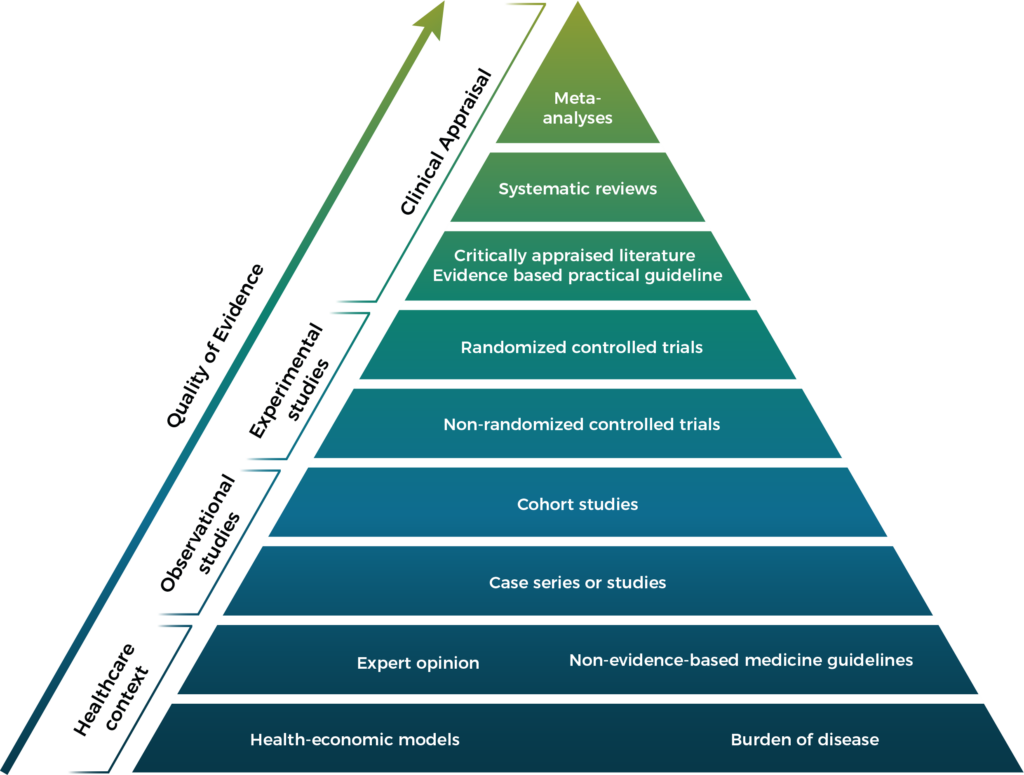
Peer-reviewed publications are the strongest form of evidence. A variety of projects can result in a peer-reviewed publication as illustrated on the pyramid below:
Quality improvement
- Progressive web applications (PWA)
- Paper or E-data-collection forms
- Digital data-collection tool
- Dashboard summaries
- Surveys, Audits
Quality improvement
Aim: To collect real-world data on clinical practice to support healthcare improvement.
Possible methods:
• Digital data-collection tool
• Progressive web applications (PWA)
• Paper or electronic data-collection forms
• Audits
• Surveys
• Dashboard summaries
Benefits: Quantification of general compliance, product usage data collection, getting customer engagement, capturing real-world practice and real-world evidence, suitable for peer-reviewed publications, and development of product evidence.
Regulatory: Compliance with international data-sovereignty and data-protection regulations. The collection of usage data for EU Medical Device Regulations.
Related work:
- Service evaluation of the impact of capnography on the safety of procedural sedation. Link
-
Capnography for monitoring of physician-led procedural sedation procedures at gastrointestinal services (video)
-
Clinical practice, monitoring, and patient safety during procedural sedation in five countries. Poster
-
Interventions and costs associated with SIVA-defined adverse events during procedural sedation in five countries. Poster
-
Patient safety during procedural sedation using capnography monitoring: a systematic review and meta-analysis. Link
- For other related work see our publications page
Environmental impact
- Environmental data management
- Reprocessing strategies
- Life-cycle assessment
- Carbon footprinting
- Waste analysis
Environmental impact
We incorporate life cycle costing approaches to evaluate the economic and environmental footprint of interventions throughout their lifespan, informing sustainable design, production, and implementation practices.
How to demonstrate the sustainability of your MedTech product
Video coming soon
Aim: To assess the full environmental implications of medical devices and healthcare products, from their creation to their disposal.
Methods:
- Life-cycle assessment
- Waste analysis
- Reprocessing strategies
- Carbon footprinting
- Environmental data management
Informs: Opportunities for reducing the environmental impact of medical devices and improving sustainability practices.
Benefits: Having an eco-friendly product can benefit HTA decisions, improve tender success, and contribute to corporate social responsibility.
Regulatory: Ensure compliance with legal frameworks and international guidelines such as EU Green Deal or the Intergovernmental Panel on Climate Change (IPCC) report.
Life-cycle assessments (LCAs) consider all inputs and outputs during the whole life cycle of a product to quantify the impact on the environment. This takes into account: raw materials for production, supply chain and logistics, and device use and maintenance, through to the final disposal of the product. The environmental impact can take many different forms, including CO2 emissions, carcinogenic effects, water, or land use. An LCA can identify the processes in a products life cycle that have the largest environmental implications. This knowledge can be used as a starting point for discussions around possibilities to reduce a products burden on the environment.
The healthcare sector generates a significant amount of waste, particularly through disposable devices. Due to strict regulations, transformation to a more sustainable practice is challenging in this sector. A waste analysis can help you identify waste prevention potentials and can also be used to compare different products or services. A waste analysis can inform decision-making on the hospital or healthcare system level.
Environmental poster (ISPOR US, 2023). Link and PDF
Environmental Life Cycle Assessment in Medical Practice: A User's Guide. Link
The environmental footprint of health care: a global assessment. Link
Healthcare Waste—a serious Problem for Global Health Link
Circular economy of the materials in the healthcare industry Link
Circular economy design of medical devices for low-resource settings Link
Reprocessing medical devices Link
A powerful, data-driven value
story for your product
Making your product standout from the crowd requires a value message with impact. With healthcare budgets being continuously stretched, increasing the perceived value of a product is more important than ever. At Coreva Scientific we help our partners to develop new value for their products or to determine the best mix of services and treatments to offer customers. From initial study design or data collection through to market access, we move your product forward with scientific evidence always at the fore.