“In the EU, medical device manufacturers are required to prepare summary of the evidence for any implantable or high-risk device.”
The Lancet. 2018;392(10146):521-530
Data identification
How to obtain data and evidence for your MedTech product
Data underpins all value messaging for products in the healthcare sector. Peer-reviewed published studies can often provide plentiful, productive data. Knowing where and how to find it is a skill. Undertaken in the right way, a literature search can provide a high level of evidence to support product value messages. Coreva Scientific runs structured and systematic literature reviews that can help uncover value for your products. These 2 options are compared below:
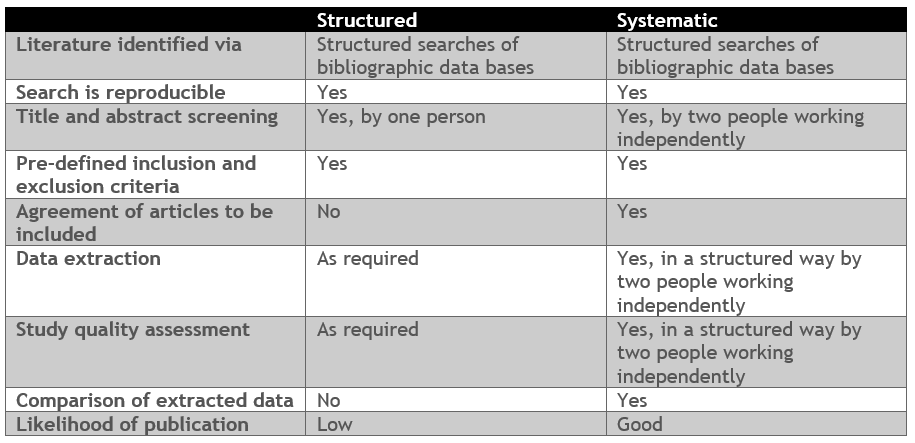
Want to understand previous regulatory decisions or to access the latest costs from a particular country. Use our expertise to help you save time in effort in pulling together the data you need for your internal decisions making.
In certain circumstances, nothing but patient data will do. Aiming to understand causes and effects or linking characteristics to costs and resource use are some examples. Quicker than doing the study yourself, data may be accessed from patient registries. Be it Europe, the US, or anywhere else in the world, we can help you identify the best available data sources for your registry study.

